New steps in the delivery of macromolecules to target cells
Recently, two research papers were published by CRIG researchers of the Ghent Research Group on Nanomedicines, presenting new steps in technology development for the delivery of macromolecules to target cells.
Gold Nanoparticle-Mediated Photoporation Enables Delivery of Macromolecules over a Wide Range of Molecular Weights in Human CD4+ T Cells – Laurens Raes
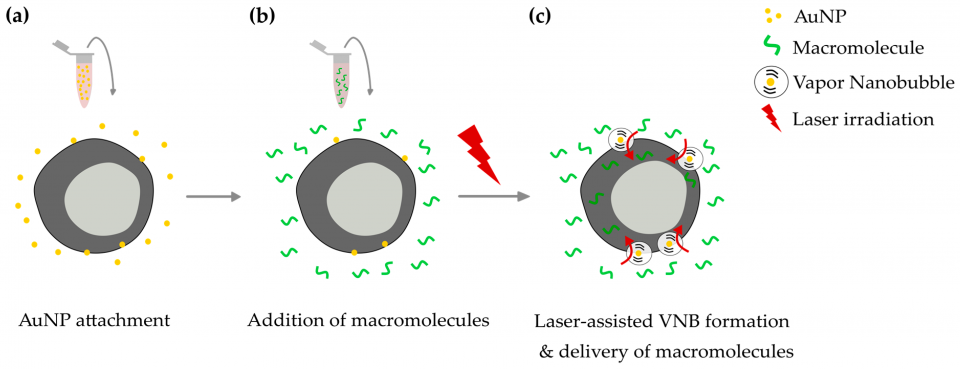
The modification of CD4+ T cells with exogenous nucleic acids or proteins is a critical step in several research and therapeutic applications, such as cancer immunotherapies. However, efficient cell transfections are not always easily achieved when working with these primary hard-to-transfect cells. While the modification of T cells is typically performed by viral transduction or electroporation, their use is associated with safety issues or cytotoxicity. Vapor nanobubble (VNB) photoporation with sensitizing gold nanoparticles (AuNPs) has recently emerged as a new technology for safe and flexible cell transfections (figure below).
In this article, Laurens Raes and colleagues evaluated the potential of VNB photoporation as a novel technique for the intracellular delivery of macromolecules in primary human CD4+ T cells using fluorescent dextrans as model molecules. They show that VNB photoporation enables efficient delivery of fluorescent dextrans of 10 kDa in Jurkat (>60% FD10+ cells) as well as in primary human CD4+ T cells (±40% FD10+ cells), with limited cell toxicity (>70% cell viability). Also, they demonstrated that the technique allows the delivery of dextrans that are up to 500 kDa in Jurkat cells, suggesting its applicability for the delivery of biological macromolecules with a wide range of molecular weights.
Altogether, VNB photoporation represents a promising technique for the universal delivery of macromolecules in view of engineering CD4+ T cells for use in a wide variety of research and therapeutic applications.
Read the full article via this link.
Nanocarrier Lipid Composition Modulates the Impact of Pulmonary Surfactant Protein B (SP-B) on Cellular Delivery of siRNA - Roberta Guagliardo
Two decades since the discovery of the RNA interference (RNAi) pathway, the first RNAi-based treatments with small interfering RNA (siRNA) drugs are being approved for several diseases, including different cancer types. Nevertheless, the widespread use of siRNA is limited by various extra- and intracellular barriers, requiring its encapsulation in a suitable (nanosized) delivery system.On the intracellular level, the endosomal membrane is a major barrier following endocytosis of siRNA-loaded nanoparticles in target cells and innovative materials to promote cytosolic siRNA delivery are highly sought after.
In previous work, the research group identified lung surfactant protein B (SP-B) as siRNA delivery enhancer when reconstituted in (proteo) lipid-coated nanogels. However, the role of the lipid component on the siRNA delivery-promoting activity of SP-B proteolipid-coated remained elusive.
In this study, Roberta and colleagues aimed to define a preferred lipid component for the SP-B siRNA delivery enhancer. Furthermore, they demonstrate that the beneficial effect of SP-B on siRNA delivery is not limited to lung-related cell types, providing broader therapeutic opportunities in other tissues as well.
Read the full article here.